Electron Microprobe Study of the Yinxu (Anyang) Bronze of Academia Sinica Collection
JEOLnews Volume 49, Number 1, 2014
Yoshiyuki Iizuka1 and Junko Uchida2
1Institute of Earth Sciences, and 2Institute of History and Philology, Academia Sinica
In this article, recent progress in broad-area photonic-crystal lasers based on photonic bandedge effect is described. It is shown that unique beam patterns can be generated by designing photonic crystal structures. Moreover, it is demonstrated that watt-class high-power, high-beamquality, surface-emitting, lasing oscillation has been successfully achieved. These results represent an important milestone for innovation in the field of lasers because it provides a route towards overcoming limitations in applications that suffer from low beam quality, which opens the door to a wide range of applications in material processing, laser medicine, nonlinear optics, sensing and so on.
Introduction
Institute of History and Philology, Academia Sinica, which was established in 1928 for modern archaeological studies, performed excavation works for 15 times in Yinxu (殷墟) of Anyang, Henan Province, the Central Plain in China. Excavation programs were suspended in 1937 due to chaotic situations. The most of excavated materials were transferred, and a large quantity of bronze objects from Yinxu has been stored in the Institute since 1949, now at Taipei.
Yinxu is the place where the oracle bone scripts were discovered and is thought to be an ancient capital in the Late Shang Dynasty (ca. 14c.BC-11c.BC), in the Bronze Age of China. The Yinxu bronzes of the Academia Sinica collection were excavated from aristocratic tombs in the Xiaotun palace area, and royal tombs in the Xibeigang area and the collection contains all kinds of bronze objects from all phases of time sequences through the Yinxu Period. Although the collection is one of the most precious and variable for study of the Bronze culture, only little amount of bronze was studied by scientific approaches. To under stand technological innovat ion of bronze casting in the East Asia, the Yinxu's materials are extremely important because it used to be the center of bronze manufacture at early Bronze Age in the East Asia. Information from the Yinxu bronzes and further comparison study of other ages, areas and technology would indicate evolution of the Bronze culture. Since 2007, the authors have launched a series of investigation of the Yinxu bronze collection using electron microprobe techniques to reveal bronze casting technology in Anyang of the Shang Dynasty. Here we report on analytical methods of ancient bronze and implications of bronze culture in the Shang Dynasty.
Ancient bronzes and sample preparations
The bronze, the first alloy of human kind, is composed of two metallic elements of copper (Cu) and tin (Sn). Bronze object was manufactured by pouring molten alloy into a mold. Melting points of Cu and Sn are approx. 1085ºC and 232ºC, respectively, and melting points decrease with increasing of Sn content in bronze. Figure 1a shows the phase diagram of Cu- Sn (tin-bronze) system. Of molten bonze within 90- 80 wt.% of Cu (10-20 wt.% of Sn), the primary solid phase of bronze is α-phase when it reached the liquidus temperature. The α-phase generates segregationsolidification (dendrite: Fig. 1b-d) during temperature falling and then secondary δ-phase appears in cooling rate of normal casting. In normal casting, crystallization of Sn-enriched phases, ε and η, does not occur because temperature falls too low to react. In other words, only dendrite α-phase and α+δ eutectic phases are observed normally in ancient bronze’s interiors as reported by Gettens[1] and Wan[2], and any phase contain less than 77 wt.% of Cu (>33 wt. % of Sn) does not exist in normal casting bronze.
It is well known that a green patina forms on bronze surface for long time of burial. The patina is composed of copper-, tin- and lead oxides and their carbonates. Without exception, the surface of ancient bronzes was oxidized. Although various analytical approaches have been applied for study of bronze chemistry such as X-ray fluorescence (XRF), XR-EDS and chemical dissolution method on the surfaces, only surface analysis is impossible to investigate its original chemistry and casting technology.
The Yinxu collection contains a large quantity (probably more than 20,000) of bronze fragments but many are not able to apply for conservation work. It is, however, still valuable and is able to choose some appropriate samples to investigate metallurgical microstructure and chemistry from cross-sections. The studied samples were selected from entire phases of the Yinxu Period (Middle Shang to Late Shang) and various usages. At least in the Han Dynasty, six kinds of usages are recognized in bronze objects, namely, vessels, instruments, arms, tools, ornaments and chariot items. To understand their chemical character of the Yinxu bronzes, ritual vessels (Jue, Ding, Zun, Gu, Pou, and Hu), arms (helmets, daggers, knives, arrow- and spear-heads) and chariot ornaments were selected for this study. Excavated helmets were only from HPKM1004 tomb in the Yinxu.
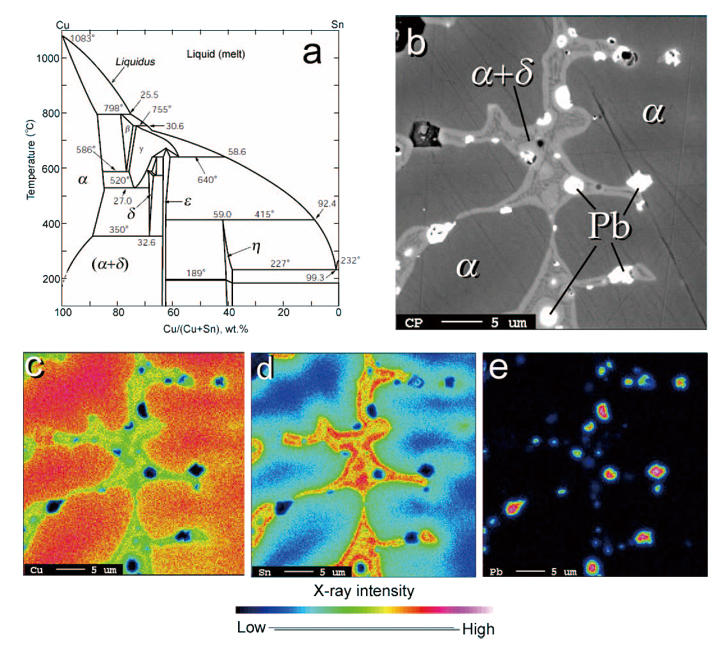
Fig. 1 Bronze system and a representative micro-structure of bronze helmet from the HPKM1004.
a) Phase diagram of copper (Cu) – tin (Sn) system in the condition of equilibrium[9].
b) Back-scattered electron micrograph. Segregated lead (Pb) particles are observed as bright (white on the image) spots
(less than few μm) in structures of α+δ eutectic phases of bronze, shown as dendrite which are surrounding the primary α
phases of bronze (shown as darker area).
c) d) and e). Elemental distribution maps of copper, tin and lead, respectively. Scale bars: 5 μm.
Analytical procedure of the Yinxu bronzes Sample preparation
To observe a cross section of bronze object, the selected fragments were sliced out a small piece (less than a few cm in size with few mm in thickness) by a micro-diamond saw. To avoid thermal and mechanical damages for metallurgical structure, slow rotation speed of the diamond saw was operated in 100 r.p.m. with distilled water for cooling during the cutting. Cleaned samples by ethanol were mounted in a cold-mounting (room temperature cured for eight hours) epoxy resin with 1-inch diameter mold and exposed surfaces were polished with diamond paste, and then finished by colloidal silica solution.
SEM
Polished cross section was initially observed by an optical microscope with the reflecting light. Then Scanning Electron Microscopes (JEOL W-SEM JSM- 6360LV and FE-SEM JSM-7100F) were used to observe metallurgical structure by back-scattered electron images, which represent mean atomic abundance by contrast in black and white images. Semi-quantitative analyses were conducted by an energy dispersive spectrometer (Oxford Instruments Ltd) used under the beam conditions of 15 kilo Volt (kV), and 0.1 nano Ampere (nA) for the acceleration voltage and beam current, respectively, in the vacuum condition of 25 Pa (Pascal). Bulk chemical compositions were determined by mean value of 10 to 20-areas of 120 μm × 90 μm (1000 times in the magnification of SEM image). X-ray counting time was for 100 seconds. The quantitative data were corrected by ZAF method with chemicalknown pure metals and synthetic alloys for Cu (copper metal), Sn (tin metal), Sb (antimony metal), Ag (silver metal), As (gallium arsenide: GaAs), Zn (zinc metal), Pb (crocoite: PbCrO4), Bi (bismuth metal), Fe-Co-Ni (NBS868 metal standard) and S (pyrite: FeS2).
EPMA
In the X-ray energy dispersive analysis on bronze, tin (Sn-L lines) is interference element for oxygen (Okα) analysis. Then, quantitative chemical analysis of copper, tin, lead and oxygen was made by EPMAs (JEOL W-EPMA JXA-8900R and FE-EPMA JXA-8500F) which equipped wave-length dispersive spectrometers (WDS). Operated beam conditions were 20 kV, 10 nA, and 5 μm de-focused beam for the acceleration voltage, beam current and beam size, respectively. The measured X-ray intensities were corrected by metal PRZ method using the standard calibration of chemical-known standard metals and oxides with the following diffracting crystals: copper metal for Cu-Kα with LiF crystal, tin-metal for Sn- Lα with PETH crystal, crocoite (PbCrO4) for Pb-Mß with PETH crystal and tin-oxide (SnO2) for O-ka with LDE1H crystal. X-ray peaks are their both upper and lower baseline X-rays which are counted for 20 and 10 s, respectively. To obtain bulk chemistry from each sample, analyzed points were randomly selected 100 to 225 points at 50 μm intervals with X-Y directions by the mapping point table conversion. Both secondaryand back-scattered electron images were used to avoid damaged and weathered areas. Then the bulk chemistry, especially bulk Cu/(Cu+Sn) ratios (Cu#), were calculated as mean values. Chemical distribution (mapping) analysis of Cu, Sn, Pb, O and some others (As and Sb) was also performed by FE-EPMA at the condition of 20 kV and 30 nA for the acceleration voltage and beam current, respectively.
Results
Metallurgical structure of the bronze’s interior
The thickness of studied Yinxu bronzes is mostly 2 to 3 mm. Many of bronzes were seriously oxidized not only at their surfaces but also in their interiors occasionally. Such samples were not appropriate to investigate their metallurgical structure. We attempted near 200 bronze fragments, but 95 samples were able to study their cross sections.
Figure 1 (b-e) shows a representative dendrite structure in the Yinxu bronze object (helmet F1 from HPKM1004) with back-scattered electron micrograph and its chemical distributions of Cu, Sn and Pb. Lead (Pb) is segregated from the bronze phases which are composed of α phase, the primary crystal phase, and α+δ eutectic phases. Pb is a fusing agent and is conducting melting point to be lowered. Pb was in molten bronze at the high temperature, but is not distributed in solid bronze.
The bronze micro-structures are divided into two types, such as dendrite and granular structures (Fig. 2). The dendrite is substantially present but granular structure is observed only 5 cases so far. For structure comparison, we study experimental bronze objects simultaneously. The dendrite is observed in normal casting bronzes, whereas granular or chemical homogeneity structure is obtained from experimental products after thermal treatment such as annealing or tempering. The results indicate that at least a kind of thermal treatment method has already applied in the Yinxu Period of the Shang Dynasty, it is very uncommon though.
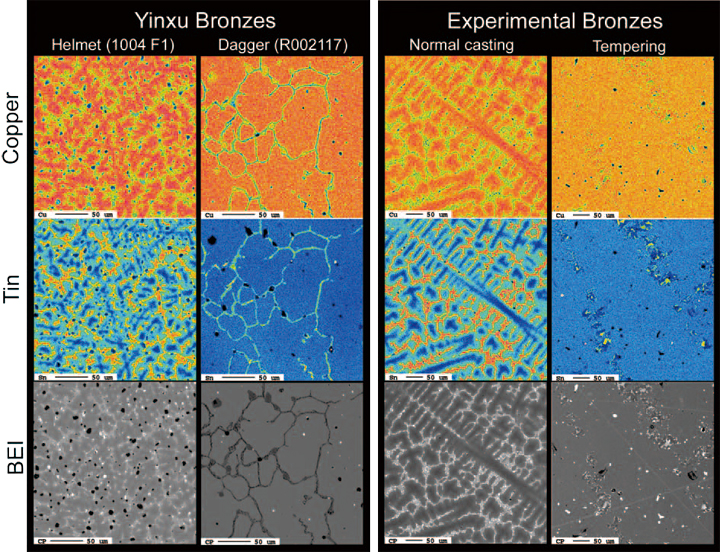
Fig. 2 Representative metallurgical structure of bronzes objects from Yinxu (left) and experimental products (right). Dendrite structure and chemical heterogeneities of Cu (tops) and Sn (middles) are observed from a Yinxu bronze object (helmet: HPKM1004 F1) and normal casting experiment (JY-4: Cu:Sn:Pb = 80:15:5, the Cu# = 0.158), on the other hand, granular structure and chemical homogeneity are observed from the interiors of dagger (R002117) and run product after tempering (JY6T: Cu:Sn:Pb = 80:15:5, the Cu# = 0.158; heat-treatment experiment: kept at 600 ºC for 24 hrs and then processed a slow-cooling). BEI: back-scattered electron micrographs. Colder (blue) and warmer (red) colors indicate lower and higher concentration of each element, respectively. Scale bars: 50 μm.
Chemical composition of the Yinxu bronzes
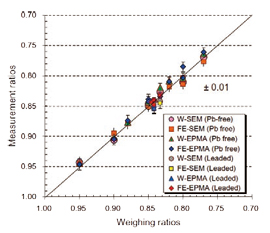
Accuracy of bulk Cu/(Cu+Sn) ratios (Cu#) of bronzes was confirmed by chemical known bronze alloys. Figure 3 shows performed results by 4-type of analysis methods. Some standards were leaded bronzes (3 to 10 wt.% in original weights). Lead (Pb) is fusing agent and behaves as volatile gas during the experiments. Thus Pb contents were expected to be decrease from the originals. However, the Cu# in the leaded bronzes were well maintained. Over all results are acceptable within 0.02 in the Cu#[3].
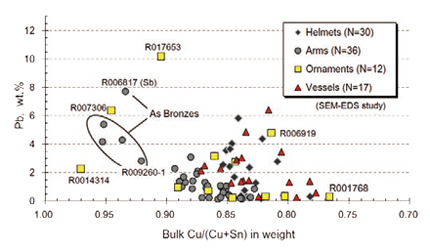
Figure 4 shows Pb content with the Cu# of the studied 95 bronzes by the usages. The most of bronzes are ranging between 0.77 and 0.89 in the Cu#, and do not contain much Pb (less than 2 wt.%). The bronzes, contained more than 0.9 in Cu#, are four (4) arsenic (As) bronzes (Fig. 5a), one (1) antinomy (Sb) bronze from the arms (Fig. 5b), and Pb-bronze (2-ornaments: R014314 and R007306) which show less Sn were identified. Excluding such bronzes (> 0.9 in Cu#), the most of studied Yinxu bronzes are shown less-content of Pb and more than 75% of the studied bronzes are shown less than 2 wt% in Pb. The mean value of the Cu# and Pb of helmets from the HPKM1004 are 0.838 and 1.56 wt.%, respectively, in the studied 30 samples. On the other hand, the #Cu of the ritual vessels ranges 0.78-0.88 which is relatively lower Cu (or higher Sn) range. Most of the vessels contain some amounts of Pb (up to 7 wt.%). In the ornaments, Cu# ranges widely from 0.77 to 0.98. A sample which highly decorated with inlaid of turquoise (R017653) shows the highest Pb content (10.5 wt.%) in this series of analysis.
In the chariot items, sample R006919 contains rather high Pb as 4.8 wt.%, and the #Cu is similar to the other ritual vessels as 0.814. On the other hand, so called a bow-shaped ornament (R001768), contains less Pb (0.3 wt.%), and the #Cu is only 0.766. It is so far the lowest value of #Cu and differs from the ratio of other arms. This is thought to be a kind of arms, but its chemical composition indicates different concern to categorize the objects. Cavities or void spaces were well observed in the interior of high-Pb bronzes generally. Less cavities are observed in the interior of low-Pb bronzes. It seems that condition of preservation is relatively better in lower Pb bronzes.
 |
 |
| Fig. 3 Analytical results of chemical-known bronze standard materials. X- and Y- axes represent weighing and measurement ratios of bulk Cu/ (Cu+Sn). W-SEM: JEOL JSM-6360LV with Oxford Si[Li] EDS; FE-SEM: JEOL JSM-7100F with Oxford SDD-EDS; W-EPMA: JEOL JXA-8900R; FE-EPMA: JEOL JXA-8500F. |
Fig. 4 Distributions of Pb contents with the bulk Cu/(Cu+Sn) ratios of studied Yinxu bronze objects shown by the usages. Diamonds: helmets from only the HPKM1004; circles: weapons: four (4) of them are identified as As-bronzes and one is As-Sb bronze (R006817); squares: decorations such chariot ornaments (3 of them are high-Cu bronzes); triangles: pots and cups with fine relieves on the surface. N: numbers of studied samples by SEM-EDS. |
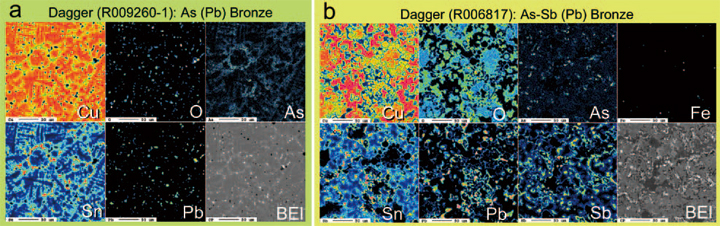
Fig. 5 a) Mapping result of As (Pb) bronze (dagger: R009260-1). Arsenic (As) distributes mostly in δ-phase of bronze but not exists in δ-phase because the solidus of Cu-Sn α-phase is too high to form Cu-As alloy. Highest value of As in δ-phase is approx.3 wt.% and bulk content of As is approx. 1wt. %. We suppose that As might be derived from copper ore mineral in nature instead of an addition because shape this dagger is expected to be the northern warriors type.
b) Mapping result of Sb-As (Pb) bronze (dagger: R006817). Antimony (Sb) is observed as segregated phase, such similar behavior as Pb and Fe (iron). As distributes mostly in δ-phase of bronze Colder (blue) and warmer (red) colors indicate lower and higher concentration of each element, respectively. BEI: electron back-scattered micrographs. Scale bars: 50 μm.
EPMA results with Oxygen analysis
Presence of oxygen in bronzes’ interior indicates condition of preservation. Thus inspection of oxygen is useful to discriminate their original chemistry for further discussion. Figure 6 shows representative results of mapping analysis on two samples, an oxidized and a well-preserved bronze helmet of helmets , from the HPKM1004. It is obviously impossible to indicate their state of the oxidation from the back- scat tered images (BEI) on both sections because the dendrites were clearly identified. However, oxygen mappings demonstrate the microdendrite structure was oxidized in the helmet-07. Quantitative spot analysis results are shown in Fig. 7. Oxidized helmets (Hel-06 and -07) shows scattered O range up to 25 wt.% and the Cu/(Cu+Sn) ratios from 1.0 to 0.3 which are inconsistent with normal casting bronze, whereas well-preserved helmets (Hel- 05 and -08) does not contain O and all Cu# range are consistent with α-phase and α+δ eutectic phase (from 94 to 72 wt.% of Cu: see Fig. 1a). The results indicate that oxidation process vary its Cu# from the original.
In the EPMA overall results, 73 bronze objects were confirmed as well-preserved samples and keep reliable chemistry to discuss its original bulk Cu:Sn ratios. Figure 8 shows di st r ibut ions of the Cu # (bulk Cu/[Cu+Sn] ratio) by usages. Each Cu# was calculated by 100-225 spots analysis by EPMA.
The helmets from the HPKM1004 (grey) are ranging between 0.80 and 0.89, and most of the helmets are between 0.83 and 0.86, and mean value is 0.843 in the Cu#. The arms (green) are also wide in range from 0.82 to 0.89 in the Cu#. The data contains various types of arms though. On the other hand, the Cu# of the ritual vessels (red) are ranging from 0.80 to 0.86. Differences in the bronze chemistry seem not clear in the variation of the time sequence through the Yinxu Period.
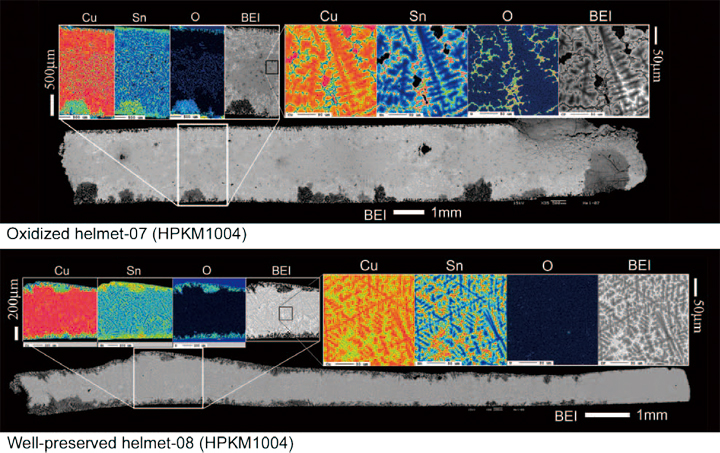
Fig. 6
Back-scattered electron micrographs (BEI) and elemental distribution maps of copper (Cu), tin (Sn) and oxygen (O). An oxidized- (upper: helmet-07: thickness 3 mm) and a well-preserved (bottom: helmet-08: thickness 1mm) bronzes from the HPKM1004. X-ray intensities were counted for 0.04 sec and 0.025 sec at intervals of 2 µ m and 0.5 µ m with the X-Y stage driving in wide (sections) and small (250 × 250 µ m) area maps, respectively.
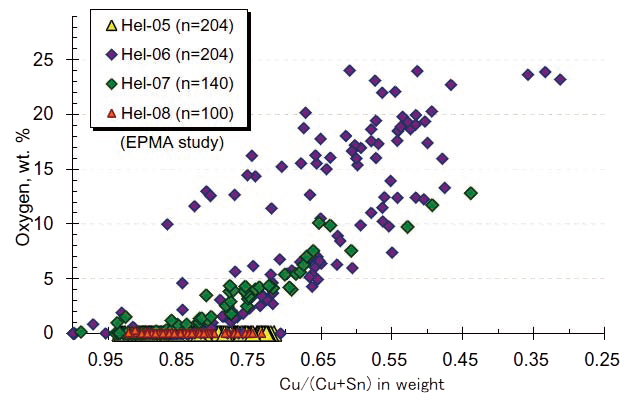
Fig. 7 Representative results of oxygen contents with the Cu/(Cu+Sn) ratios from the interiors of the bronze helmets from the HPKM1004 by EPMA spot (quantitative) analysis. Diamonds: oxidized bronzes (Helmets-06 and -07). Triangles: wellpreserved bronzes (helmets-05 and -08). n: numbers of analytical spots.
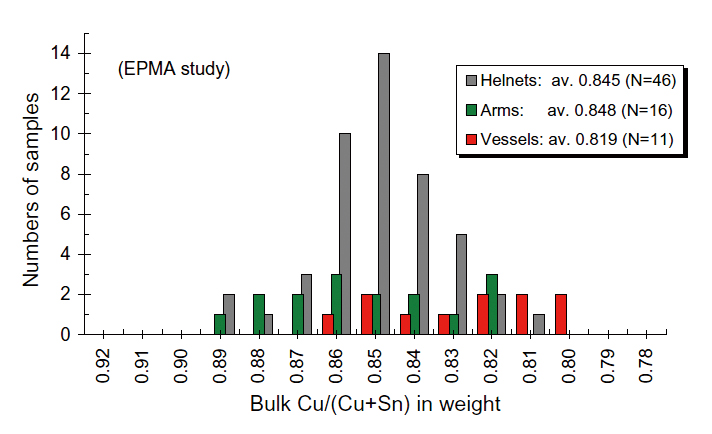
Fig. 8 Distributions of the bulk Cu/(Cu+Sn) ratios by usages. Grey: helmets from only the HPKM1004; green: arms include daggers, knives, arrow- and spear-heads); red: vessels. av.: average in the bulk Cu/(Cu+Sn) ratios in weight. N: numbers of studied samples by EPMA.
Discussion
It is well studied about physical properties of Cu-Sn alloy in the modern metallurgy that the color, the Brinell hardness, the tensile strength, and the elongation of bronze vary with ratios of Cu:Sn (Fig. 9). In general, bronze is getting harder with increasing of Sn content from 15 wt.% but it is getting brittle because the tensile strength and the elongation are significantly decreasing with Sn content more than 20 wt .%. It seems that bronze gains most toughness around 85-80 wt.% of Cu (15-20 wt.% Sn). Estimated viscosity of molten bronze is also shown in Fig. 9 that viscosity is decreasing with increasing of Sn content[4].
So far we obtained 73 bronze chemistries and 46 data of them were helmets from the HPKM1004. As shown in Fig. 8, the #Cu of helmets shows relatively uniformed and most of helmets distribute between 0.83 and 0.86, and mean value is 0.843. Their #Cu ratios seem comparable to high toughness range of bronze. On the other hand, the Cu# range of the ritual vessels is slightly Sn-enriched and Pb as well (see Fig. 4). The vessels are usually decorated with fine relieves on the surface. These phenomena indicate that the Sn and Pb were added intentionally in order to increase the viscosity of molten bronze for casting, which might be easy to pour into fine decoration molds. Various kinds of arms were studied but some of arms seem ritual objects with surface decoration instead of real weapons. It might be the reason that the Cu# of arms distributes in wider range from 0.82 to 0.89. From these circumstances, it is likely that craftsmen already understood characters of bronze at the time
In the ancient Chinese classic of the Zhou Dynasty of Rites of Zhou < 周礼考工記 Zhou Li Kao Gong Ji>, the Six Formula of mixture ratios < 金有六 斉 Liu Qi> were standardized for different usages of bronze. It has debated that description was in the 4th to the 3rd Century BC on the basis of the knowledge of the 9th to the 7th Century BC which is represented of the period of Zhou Dynasty. According to the descr ipt ion, it is thought that di f ference of the bronze alloy components (ratios of Cu:Sn) might be controlled since long time ago in China. However it has not been confirmed with chemical compositions of ancient bronzes.
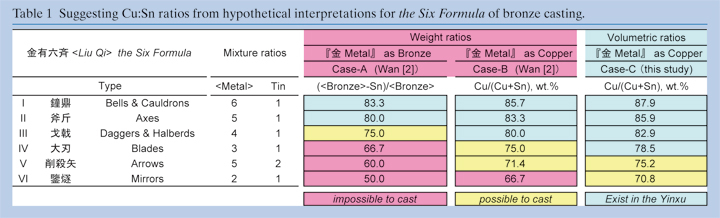
Wan[2] proposed 2-ways interpretation of the Six Formula based on weighing ratios, shown as cases -A and -B in Table 1. Because a term of copper was not present at the time, the ancient sentences in the Six formula were described bronze mixture ratios with “Metal” and “Tin”. He assumed “metal” in case of bronze (case-A) and copper (case-B) and estimated the six mixture ratios in weight in the Cu# from 83.3 to 50 wt.% in case-A, and from 85.7 to 66.7 wt.% in case-B.
In modern metal lurgy, it is well known that bronze which contained less than 66.7 wt.% of Cu (more than 33.3 wt.% of Sn) is unable to cast. In the metallurgical point of view, a bronze which is composed of only δ phase (68.2-66.8 wt.% of Cu) is also not exist. The world most highest Sn (lowest Cu) bronze object has been recorded as 32.6 wt.% of Sn (67.4 wt.% of Cu) from Kerala, South India[5]. As shown in the results, most of the Yinxu bronzes are constructed by α and α+δ phases. The lowest Cu# is 0.783. On the other hand, the highest Cu# of 83.3 and 85.7 wt.% in case -A and -B, respectively. However there are not reliable because the wellpreserved bronzes are dispersed up to the range of 0.89 in the Cu#. Therefore it is suggested that Wan’s 2-hypotheses are inconsistent in reality.
Hori[6] investigated the ancient balance-weights from the Ancient Central Asia and it was confirmed that the Central Asian weight system was established around 4000BC and is the oldest in the world. On the other hand, Chinese weight system might be established in later than 1000BC which might be comparable to Post-Shang Dynasty. Qiu et al. [7] interpreted ancient Chinese articles that volume unit by the decimal system was already established in the Pre-Qin Period (by 221BC). They also pointed out in historical point of view that the appearance of the weight unit is later than the establishment of units of length and volume. Two Pb ingots were excavated from the Xiaotun E-16 pit at Yinxu [8] but any weighing and balance-weights tool were not discovered yet from Anyang. It is supposed that, therefore, weighing system was not established yet in the Yinxu Period.
Volumetric system is another measurement way. The density of Cu and Sn (ß-Sn) are 8.94 and 7.365 (g/cm3) respectively. Thus the mass (or weight) of Cu and Sn are different in even the same volume. Assuming that mixture ratios of the Six Formula are based on the volumetric ratios, their weight ratios are suggested from 88 to 71 wt. % within few % steps in the Cu# (case-C in Table 1). From the analytical results, the #Cu of helmets from the HPKM1004 are relatively uniformed within 3 wt.% and these ranges are also reflected to the physical property of bronze. Further the suggested range of the Cu# from 88 to 71 wt.% of Cu might be reliable for casting. Thus it is likely that volumetric hypothesis is probable.
In types I and III of the Six Formula instruct the ratio for Bells & Cauldrons (Cu6:Sn1=88 wt.% of Cu) , and daggers (Cu4 :Sn1= 83 wt.% of Cu) , respectively, which are represented vessels and arms. Thus it indicates that the vessels are enriched in Cu than the arms, however, the analytical results showed cont radict ion that the arms are enr iched in Cu than the vessels. It is also inconsistent with physical property of bronze.
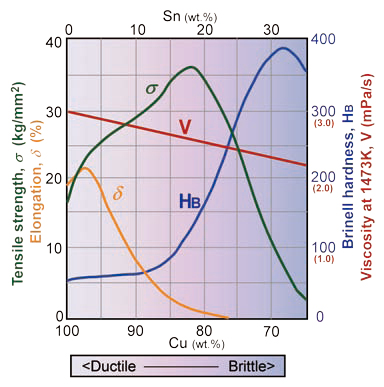
Fig. 9 Physical properties of bronze as functions of chemistry. σ : tensile strength [2]; δ : elongation; HB: hardness (Brinel Number) [10], and V: estimated viscosity of molten bronze at temperature of 1470K[4].
Conclusion
We attempted near 200 bronze objects from the Yinxu (Anyang) in the Academia Sinica collection to invest igate thei r metal lurgical st ructure and chemistry. Most of the Yinxu bronzes are tin (Sn)- bronze with a little amount of lead (Pb). Bulk Cu/ (Cu+Sn) rat ios (Cu # in weight) of each bronze fragment were calculated by average of 100 to 225 EPMA quantitative (spots) analysis with oxygen and lead, followed by semi-quantitative analysis by SEM-EDS . The Cu# from non-oxidized interior represents its original Cu#, whereas the ratios could significantly be shifted after oxidation. We confirmed that 73 samples of arms (helmets, daggers, arrowand spear-heads) and vessels were well preserved. An overall result indicates that the original Cu# ranges from 0.79 to 0.89. No variation is verified in time sequence during the Yinxu Period. By the usages, the Cu# of helmets are between 0.84 and 0.89, and 0.845 in average, and it is relatively uniformed when compared with other various usages. The most of helmets do not contain much Pb (< 2 wt. %), and less cavities are observed in the interior. Physical property of bronze is varied by their Cu#, and helmet chemistries are fit its toughness chemical range. The vessels show relatively lower in Cu# (high-Sn bronze) and contain some amount of Pb (up to 5-6 wt. %). In the contrast with the helmets, the vessels are usually decorated with fine relieves on the surface. Since lower Cu# (or higher Sn content) and addition of Pb reduce viscosity of molten bronze, it might be easy to cast with fine decoration molds. It is suggested from the results of chemical analysis that the Cu# of bronzes was intentionally controlled by purpose of usages within few % in the Yinxu Period. Based on this series study of the Yinxu bronzes, we proposed a hypothetical interpretation of the Six Formula by volumetric ratios might be probable, instead of the weight-base interpretation. However, the Cu# of the Yinxu bronzes are inconsistent with the ratios which were described in the ancient Chinese classic.
Acknowledgments
We thank Dr. Kwang-tzuu Chen and Ms. Yuyun Lin of Institute of History and Philology for thei r kind suppor t. Professors Haruhisa Mi fune and Takekazu Nagae of University of Toyama are appreciated to provide experimental bronze samples and valuable di scus sion. We thank Ms. Ya-t ing Hsu, Mr. Yu-shiang Wang and Ms. Hui-ho Hsieh of Institute of Earth Sciences for their technical support of bronze analysis. This study is supported by National Science Council (Taiwan) and the Institute of History and Philology of Academia Sinica.
References
[ 1 ] Gettens R.J. 1969 The Freer Chinese Bronzes Volume I I Technical Studies . Smithsonian Institution Freer Gallery of Art, Oriental Studies, No.7. Washington DC.
[ 2 ] Wan Chia-pao (1970) A preliminary report on the metallographic examinations of Shang bronze helmet s. Institute of History and Philology Academia Sinica Special Publications, No.60. pp.4 8. Taipei (in Chinese with an English summary).
[ 3 ] Iizuka Y., J. Uchida (2013) Chemical compositions of Yinxu (Anyang) bronze objects in the Academia Sinica collection and its implications for Ancient Chinese Casting techniques. Bulletin of Japan Society of Chinese Archaeology, 13:23-47 (in Japanese with Chinese abstract).
[ 4 ] Kozlov L.Y., L.M. Romanov, N.N. Petrov (1983) Predict ion of mult icomponent metal melt s viscosity. Izvestiya Vyssh. Uch. Zav., Chernaya Metallurgiya, 3:7-11.
[ 5 ] Mifune H. (2010) Comparison of the manufacturing technology of high-tin bronze tools in modern Asia. In Asian high-tin bronzes: Production technology and regional characteristic. pp.125-135 (ISBN 978-4-9905066-1-2).
[ 6 ] Hori A. (2007) Reconsideration of the Weight System of the Ancient Central Asia. Bulletin of the Society for Near Eastern Studies in Japan (Nippon Oriento Gakkai), 50(1):30-32 (in Japanese with English abstract).
[ 7 ] Qiu Guangming, Qiu Long, Yang Ping (2001) Unit of Weight (Chapter 4) in History of Chinese Technology. Science and Technology Press, pp.25- 31. Beijing (in Chinese).
[ 8 ] Chen Kwang-tzuu (1991) Analysis and study on Lead Ingots from Yinxu. In Archaeology and Historical Culture. Commemoration of the Eightieth Anniversary of Kao Ch'u-hsun, pp.355- 388. Cheng-Chung Book. Taipei (in Chinese).
[ 9 ] Massalski B.T. (Editor-in-chief) (1990) Binary Alloy Phase Diagram: Second edition. National Institute of Standards and Technology, Library of Congress Cataloging in Publications Data, USA (ISBN-10: 0-87170-405-6).
[10] Scott D.A. (1991) Metallography and microstructure of ancient and historic metals. The J. Paul Getty Museum, pp.155. Los Angels (ISBN 0-89236-195-6).
