定量補正
定量補正
quantitative correction
[目次:分析]
電子線照射によって発生する特性X線を用いて、未知試料(測定したい試料)中の元素の定量分析を行う際に用いる補正。未知試料と標準試料(構成元素の質量濃度が既知)から発生する特性X線の強度比(Kレシオ=CU/CStd)を用いて算出された未知試料の第一近似濃度に補正を行い、真の濃度を決定する。
未知試料と標準試料では構成元素とその存在比が異なるために、測定した強度比(Kレシオ)は未知試料と標準試料の濃度比に等しくはならず、補正が必要である。補正因子Gは、次に示す三つの補正からなる。1) 特性X線の発生効率(原子番号補正GZ)、2) 発生した特性X線が試料内を通過する際の吸収(吸収補正GA)、および 3) 他の元素に起因する蛍光X線の効果(蛍光補正GF) (図1)。これらの補正因子を計算する手法として、ZAF補正やφ(ρz)法がある。
定量補正計算の流れを図2に示す。補正因子Gを算出するには、まず未知試料からの全ての特性X線を測定し、未知試料の構成元素(N = A, B, C,…)を特定する。以下に、上に述べた補正を行って未知試料中の元素の質量濃度の求め方について説明する。
元素A に対して測定から得られたKレシオKA =CAU/CStdAに、標準試料中の元素Aの質量濃度CStdAを掛けることで、未知試料中の元素Aの質量濃度の第一近似濃度CA0を算出する。未知試料の元素 B, C,… に対しても同様のことを行い、第一近似濃度 CB0, CC0, …を算出する。未知試料を構成する全元素の第一近似濃度CN0を用いて補正因子GN0を計算で求める。第一近似濃度CN0(= CStdN x KN)にGN0を掛けて第二近似質量濃度CN1を求める。次に、補正した全ての質量濃度CN1を用いてGN1の値を求め、 GN1をCN0に掛けることで CN2を得る。この操作を繰り返して、算出した質量濃度 CNi+1 の値がその前の値 CNi に近くなったときに、その値を真値とする。
KレシオやCN0を求める方法として、①標準試料を用意して特性X線強度を実測するスタンダード定量、②理論計算やEDSメーカーが用意したリファレンスデータを用いるスタンダードレス定量、の2種類がある。
未知試料と標準試料での構成元素の平均原子番号の差が大きく異なると、特性X線の発生効率や試料内での吸収の違いが大きくなるため、補正量は大きくなる。一般的には吸収補正の効果が最も大きく、次いで原子番号補正、蛍光補正の順となる。特に20kVや30kVといった高い加速電圧では、電子線が試料内のより深い位置まで侵入し特性X線を発生させるので、特性X線が試料内を通過する距離が長くなるため、吸収補正が重要となる。蛍光補正は特定の元素の組み合わせ以外は影響が小さいため無視できることが多い。吸収補正、原子番号補正、蛍光補正の詳しい説明はZAF補正の項目を参照のこと。
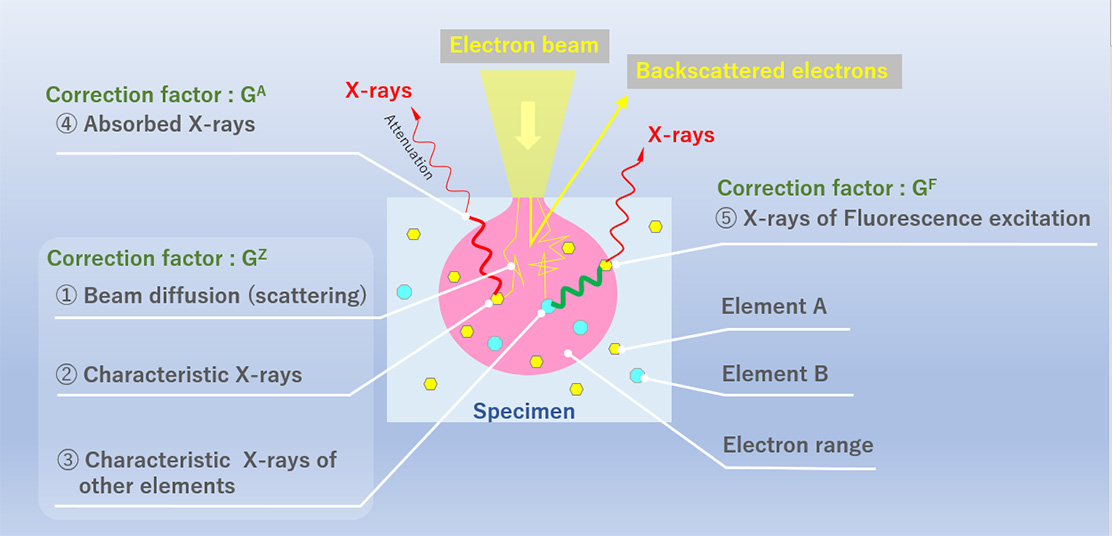
図1 定量補正に使用する三つの補正因子
試料に入射した電子の一部は、試料を構成する原子と弾性的・非弾性的に散乱されて(①)、一部は反射電子として試料外に放出される。試料内に侵入した電子は連続X線を発生させつつ、元素Aの特性X線を発生させる(②)。また、試料中の別の元素Bの特性X線を発生させ(③)、そのエネルギーを失う。このような散乱過程における特性X線発生効率の標準試料と未知試料での違いを原子番号補正GZとして補正する。
発生した特性X線の一部は試料に吸収される。吸収の大きさは構成元素とその存在比により異なるため、標準試料との違いを吸収補正GAとして補正する(④)。
また、他の元素Bの特性X線(③)や連続X線により、元素Aの特性X線と同じエネルギーのX線(蛍光X線)が発生する場合(⑤)がある。蛍光X線によるX線強度の標準試料との違いを蛍光補正GFとして補正する。
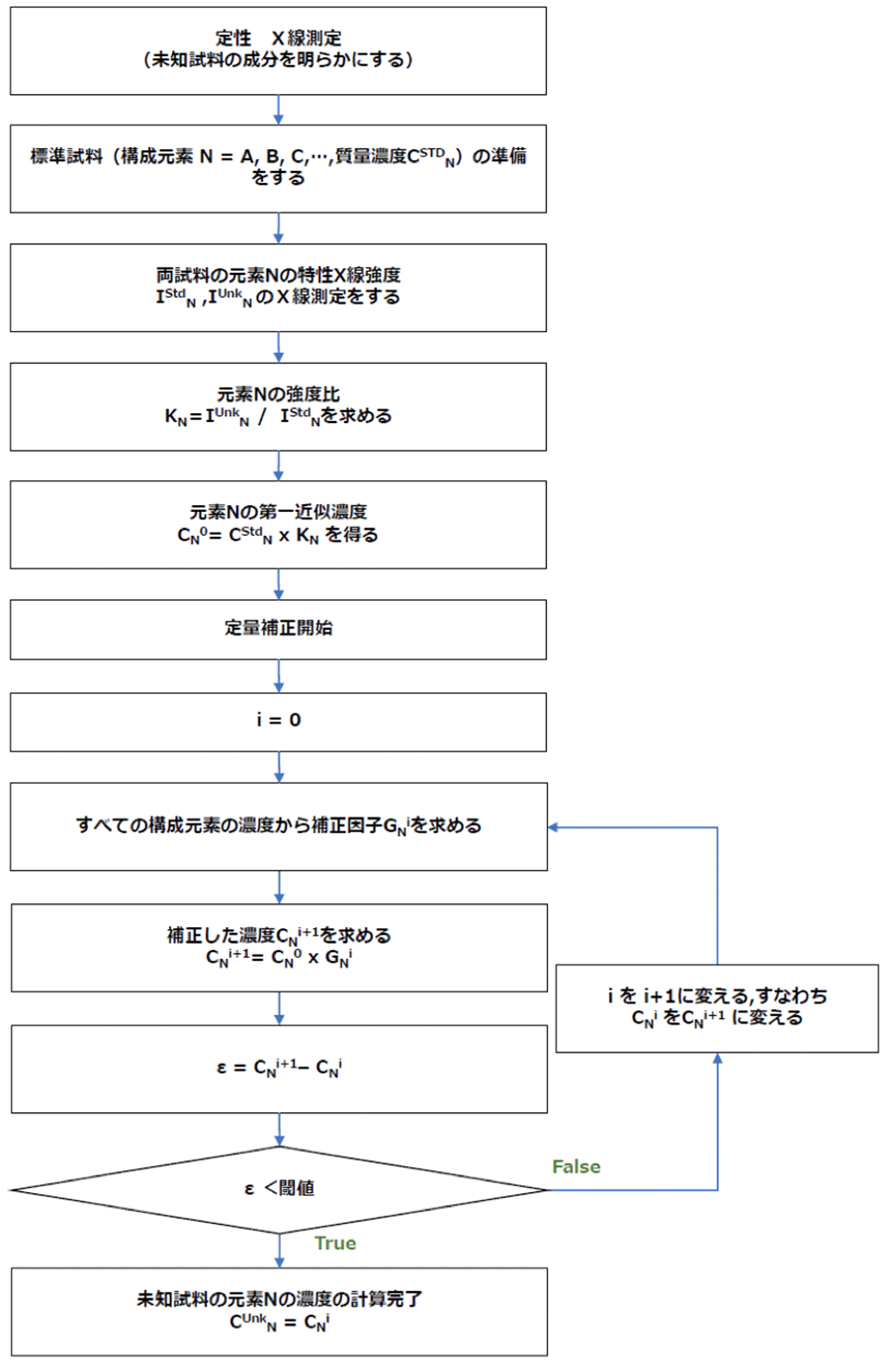
図2 未知試料の質量濃度への定量補正計算の流れ(スタンダード定量の場合)
A correction to obtain the correct concentration values of the constituent elements in an unknown specimen using the characteristic X-rays generated by electron beam irradiation. The correction is applied to the first approximate concentration value, which is calculated using the intensity ratio (K ratio = CU/CStd) of the characteristic X-rays from the unknown specimen and the standard specimen (mass concentrations of the constituent elements are known).
The constituent elements and their abundance ratio of the unknown specimen are different from those of the standard specimen. As a result, the measured K ratio is not equal to the concentration ratio of the unknown specimen to the standard specimen. Thus, corrections are needed to obtain the correct concentrations of the unknown specimen. The correction factor G is composed of the following three corrections: 1) Atomic number correction GZ due to the difference of characteristic X-ray generation efficiencies, 2) Absorption correction GA due to the difference of absorption of the characteristic X-rays when they run in the specimen and 3) Fluorescence correction GF due to the difference of fluorescence X-ray generation efficiencies (Fig. 1). Methods for calculating these correction factors include the ZAF correction method and the Phi-Rho-Z method (φ(ρz) method).
The workflow of the quantitative correction (in standard quantification) is shown in Fig. 2. To obtain the correction factor G, first, all the characteristic X-rays generated from the unknown specimen are measured and the constituent elements (N = A, B, C,…) of the specimen are identified. In the following, we explain how to obtain the mass concentrations of the elements in the unknown specimen with the corrections described above.
The first approximate concentration value CA0 of element A in the unknown specimen is calculated by multiplying the measured K-ratio KA = CAU /CStdA by the mass concentration CStdA of element A in the standard specimen. By the same procedure, the first approximate concentrations CB0, CC0… of elements B, C...in the unknown specimen are calculated. Then, the correction factor GN0 (N=A, B, C…) is calculated using the first approximate concentrations CN0 of all the constituent elements contained in the unknown specimen. The second approximate mass concentration CN1 is calculated by multiplying GN0 by the first approximate concentration CN0 (= CStdN x KN). Next, using the corrected mass concentration CN1, GN1 is obtained, and CN2 is calculated by multiplying CN0 by GN1. By repeating this procedure, when the calculated mass concentration CNi+1 becomes very close to the previous CNi, the value is taken as the true value.
There are two methods for determining the K ratio and CN0: (1) standard quantification, in which a standard specimen is prepared and the characteristic X-ray intensity is actually measured, and (2) standard-less quantification, in which a theoretical calculation or reference data prepared by the EDS manufacturer is used.
When the difference in the average atomic number of the constituent elements is large between the unknown specimen and the standard specimen, the correction amount becomes large because the difference of the characteristic X-ray generation efficiency and of the absorption in the specimen becomes large. In general, the effect of absorption correction is largest, followed by the atomic number correction and the fluorescence correction. In particular, when the accelerating voltage is high (e.g. 20 kV, 30 kV), the electron beam penetrates deeper into the specimen while generating the characteristic X-rays. Thus, the X-rays run a longer distance in the specimen, and then, the absorption correction becomes important. Fluorescence correction can often be neglected because the fluorescence effect is small except for the combinations of specific elements. For the detailed explanations of the absorption correction, atomic number correction and fluorescence correction, refer to the term “ZAF correction”.

Fig. 1 Three correction factors used for quantitative correction
A part of electrons incident into the specimen is scattered elastically and inelastically with the constituent atoms (elements) (①). Some electrons are backscattered and emitted outside the specimen as reflected electrons. The electrons penetrating into the specimen generate continuous X-rays and the characteristic X-rays of element A (②). The penetrating electrons also generate the characteristic X-rays of element B (③). In this inelastic scattering process, these electrons lose their energy. The difference in the characteristic X-ray generation efficiency between the unknown specimen and the standard specimen in such scattering process is corrected as the atomic number correction factor GZ.
A part of the generated characteristic X-rays is absorbed by the specimen. Since the magnitude of absorption for the characteristic X-rays depends on the constituent elements and their abundance, the difference between the unknown specimen and the standard specimen is corrected by the absorption correction factor GA (④).
In some cases, the characteristic X-rays of element A are excited by the characteristic X-rays of the other element B (③) or by continuous X-rays, and the X-rays of the same energy as the characteristic X-rays of element A (fluorescence X-rays) are generated (⑤). The difference in the fluorescence X-rays between the unknown specimen and the standard specimen is corrected by the fluorescence correction factor GF.
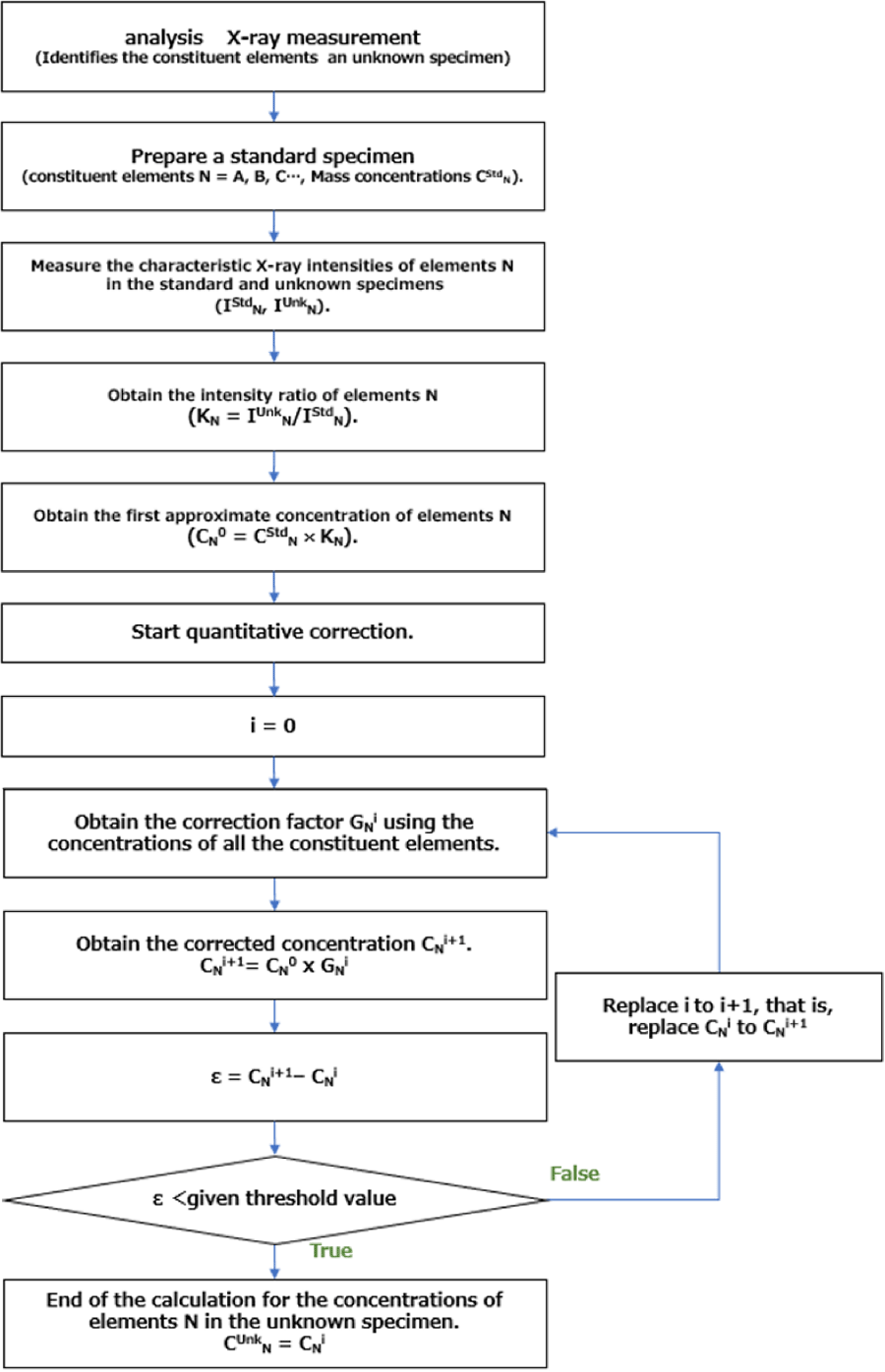
Fig. 2 Workflow of quantitative correction of constituent-elements concentrations in an unknown specimen.
関連用語から探す
説明に「定量補正」が含まれている用語